Welcome to the world of organic chemistry, which is full of molecular combinations of atoms that seem obscure at first glance but hold relevance. One of such organic compounds is hcooch ch2 h2o, which initially seems cryptic, but the triad carries a fascinating interplay or a synergistic blending among organic molecules like formic acid (HCOOH), water (H₂O), and the methylene bridge (CH₂). So without any delay, let’s explore it in the article.
This article of Vlineperol gives you an understanding of molecular insight, core principles related to organic chemistry about hcooch ch2 h2o, and also its practical applicability, no matter whether you are a chemist, professor, or student, to read the beauty of organic chemistry encapsulated within hcooch ch2 h2o in a detailed manner. Seems fascinating and makes you eager to learn more about it, right? Stay with me in the article to gain insight into it.
Understanding the Components of hcooch ch2 h2o
To better understand about hcooch ch2 h2o it is significant to understand the key components that are embedded within it.
| Components | Molecular weight | Density | Boiling point | Usage |
| Formic Acid (HCOOH) | 46.03 g/mol | 1.22 g/cm³ | 100.8°C | Act as an agent of a reduction reaction. |
| Methylene (CH2) | 14.03 g/mol | Highly reactive and unstable nature, having no stable density under standard conditions | -92.2°C | Used in organic reactions and also in polymer synthesis. |
| Water (H2O) | 18.02 g/mol | 1.00 g/cm³ | 100°C | Universal solvent assists in the hydrolysis reaction and also facilitates proton transfer. |
How Methyl Formate Hydrolyzes- Let’s understand it
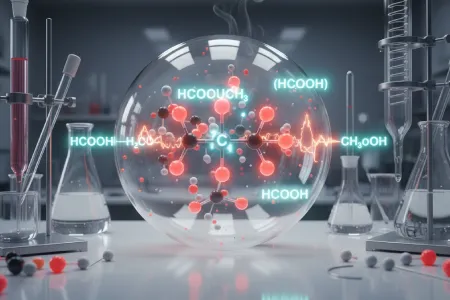
The hydrolysis of Methyl Formate is becoming prominent due to the molecular interaction among HCOOH, CH2, and H2O. Methyl formate (HCOOCH₃) is being hydrolysed in aqueous solution in the presence of water to give rise to methanol (CH₃OH) and formic acid (HCOOH).
| HCOOCH₃ + H₂O → HCOOH + CH₃OH |
This reaction is an example of a “nucleophilic substitution reaction”. The reaction undergoes the following steps during the process of organic reaction under acidic conditions, as mentioned below:
During the hydrolysis of methyl formate, the electrophile nature of the compound, namely the carbonyl compound, is raised. Nucleophilic attack led by a water molecule gives rise to intermediate formation. After the hydrolysis reaction, methanal and formic acid are produced as the end reaction products.
Applications of hcooch ch2 h2o in industry and laboratory
Practical industry-specific applicability
- Formic acid acts as a dye fixative, guaranteeing long-lasting, vibrant color.
- Latex coagulation tailored by formic acid gives rise to rubber production.
- The elasticity and tensile strength are provided during the resin and rubber production under the role of polymer synthesis reaction.
Laboratory application
- Creating a platform for a catalyst-mediated stable reaction.
- Resin has been synthesized by the use of monomers bearing CH2.
- Polymer growth optimisation by methylene intermediaries.
Environmental and safety considerations with hcooch ch2 h2o
Working with hcooch ch2 h2o to be taken with extra caution, following safety protocol, and adhering to mitigate hazards or any risky consequences. Methyl Formate is causing irritation that needs to be kept in ventilated areas to mitigate this. Goggles and protective hand gloves help to protect us from the corrosive nature of formic acid. Methanol is, by nature, toxic and needs to be kept away from us to avoid skin contact and inhalation.
Final thought
In a nutshell, the organic compound, namely HCOOCH CH2 H2O, looks like a simple organic compound, but its industry relevance and research applicability make it greatly relevant. From hydration to esterification, the compound holds paramount relevance in organic chemistry that is beyond description.